Reaction Kinetics and Reaktion Mechanisms
Adequate modeling of combustion systems is very complicated because of the complexity of combustion process, large number of chemical reactions and species, fast changes in temperature, density and volume often followed by turbulence and complex geometry.
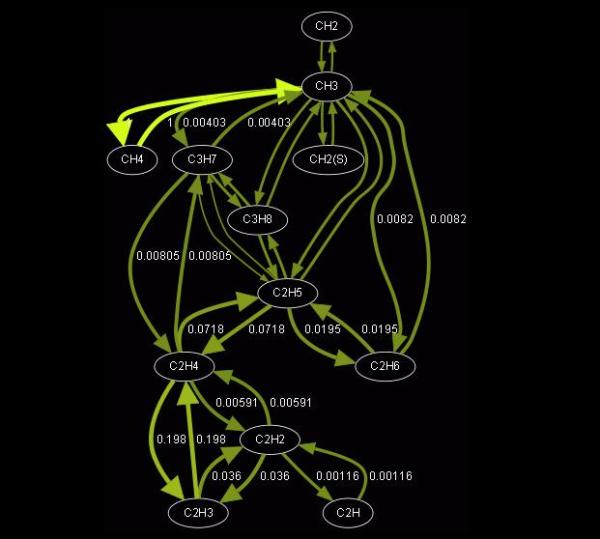
A detailed reaction mechanism is a collection of elementary chemical processes (often referred to as elementary steps or elementary reactions) that occur on a molecular level, describing the overall chemical process. Detailed reaction mechanisms for combustion of complex hydrocarbons commonly used in practice, as well as in numerical simulations, contain large amount of species, therefore a large number of theoretically possible elementary reactions among them. Under specific conditions and fixed physical parameters (e.g. pressure, temperature and mixture composition), it is reasonable to assume that overall chemical process will follow a less branched reaction path involving much less reactions and species than those predicted by a detailed reaction mechanism. Therefore, it is imperative to develop different techniques to create reduced chemical kinetic mechanisms that approximate the results of detailed chemical kinetic descriptions, over a range of conditions using fewer species, and thus less CPU time and memory.
Selected Publications
- J. T. Lipkowicz, I. Wlokas, A. M. Kempf, Analysis of mild ignition in a shock tube using a highly resolved 3D-LES and high-order shock-capturing schemes, Shock Waves (2018) 1-11. LINK
- Janbazi, H., Hasemann, O., Schulz, C., Kempf, A., Wlokas, I., Peukert, S., Response surface and group additivity methodology for estimation of thermodynamic properties of organosilanes, International Journal of Chemical Kinetics (2018) 1–10.
- Sikalo, N., Development and Application of a Genetic Algorithm-based Tool for the Reduction and Optimization of Reaction Kinetic Mechanisms, PhD Thesis (2017). PDF
- Sikalo, N., Hasemann, O., Schulz, C., Kempf, A., Wlokas, I., A Genetic Algorithm–Based Method for the Optimization of Reduced Kinetics Mechanisms, International Journal of Chemical Kinetics 47:11 (2015) 695-723.
- Sikalo, N., Hasemann, O., Schulz, C., Kempf, A., Wlokas, I., A Genetic Algorithm‐Based Method for the Automatic Reduction of Reaction Mechanisms, International Journal of Chemical Kinetics 46:1 (2014) 41-59.
- Wlokas, I., Faccinetto, A., Tribalet, B., Schulz, C., Kempf, A., Mechanism of Iron Oxide Formation from Iron Pentacarbonyl‐Doped Low‐Pressure Hydrogen/Oxygen Flames, International Journal of Chemical Kinetics 45:8 (2013) 487-498.
- Wiggers, H., Fikri, M., Wlokas, I., Roth, P., Schulz, C., Synthesis of tailored nanoparticles in flames: Chemical kinetics, in situ diagnostics, numerical simulation, and process development, In Nanoparticles from the Gasphase, Springer Berlin Heidelberg (2012) 3-48.
- Hardt, S., Hamid, A., Weise, C., Wlokas, I., Wiggers, H., Schulz, C.,Experimental and numerical investigation of flame spray assisted TiO2 synthesis from titaniumtetraisopropoxide, Proceedings of the 2011 AlChE Annual Meeting Conference (2011) 16-21.
- Staude, S., Hecht, C., Wlokas, I., Schulz, C., Atakan, B., Experimental and numerical investigation of Fe (CO) 5 addition to a laminar premixed hydrogen/oxygen/argon flame, Zeitschrift für Physikalische Chemie International journal of research in physical chemistry and chemical physics 223(4-5) (2009) 639-649.
- Wlokas, I., Staude, S., Hecht, C., Atakan, B., Schulz, C., Measurement and simulation of Fe-atom concentration in premixed Fe (CO) 5-doped low-pressure H2/O2 flames, Proc. of the 4th European Combustion Meeting (2009).