Research
Molecular clips and tweezers against pathological protein aggregation:
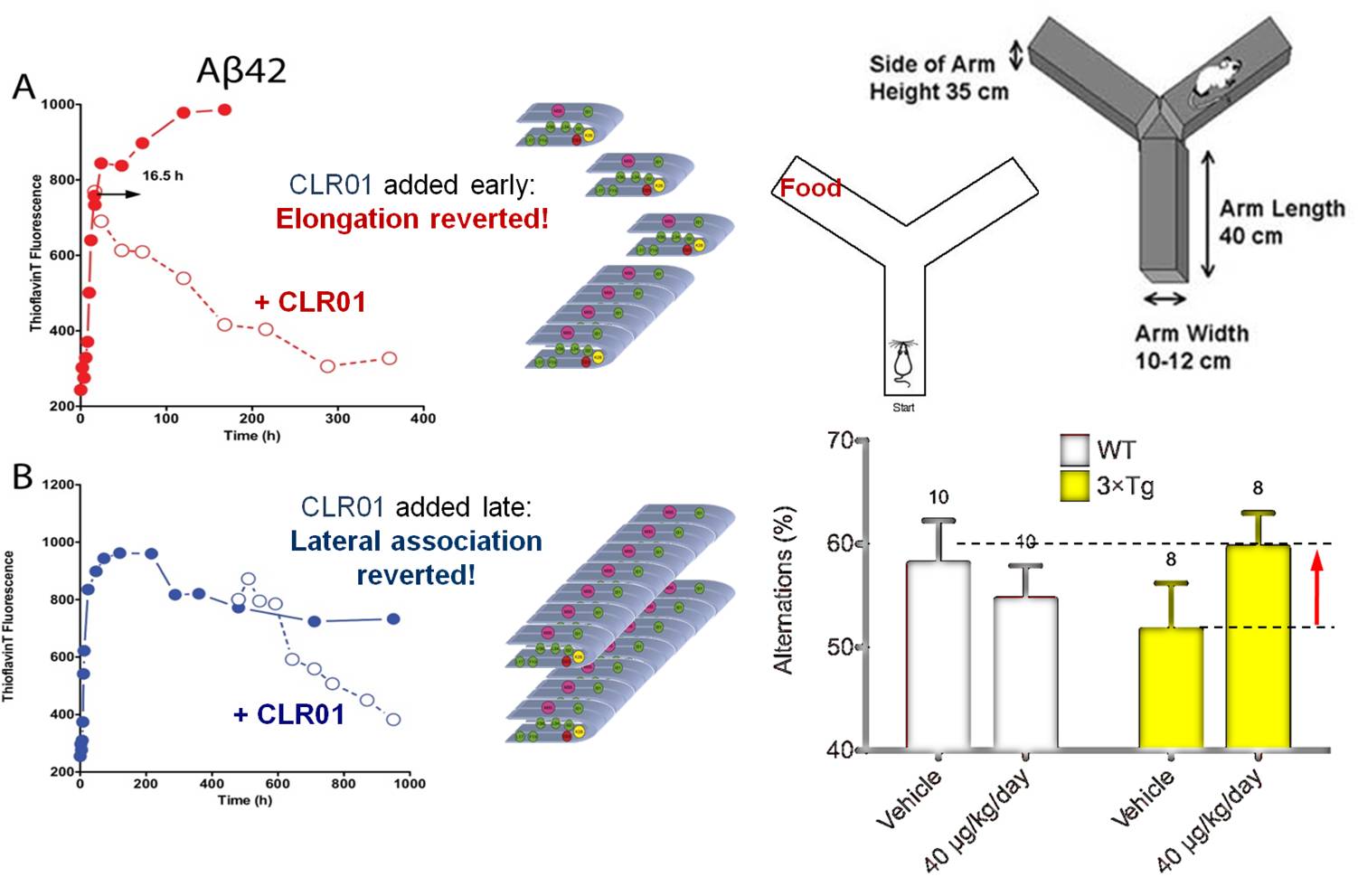
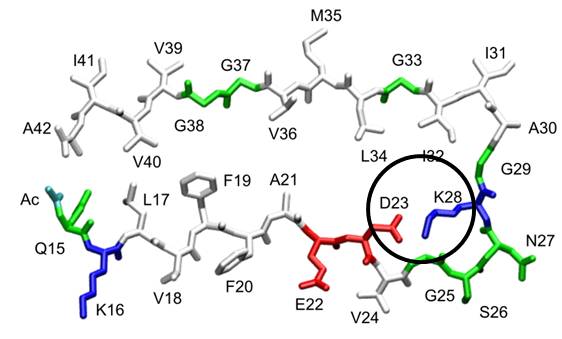
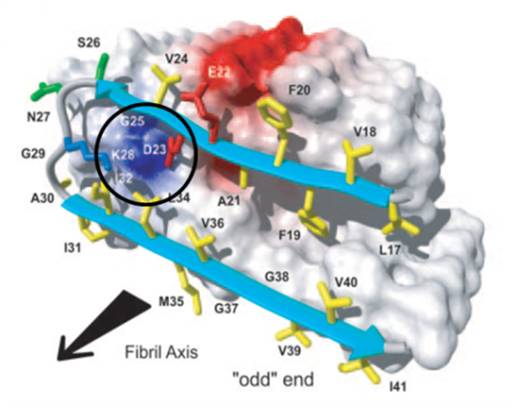
Misfolding of numerous peptides and proteins often involves critical lysine residues: thus the Alzheimer’s peptide is initially forced into a U-shaped conformation by an internal salt bridge between Asp-23 and Lys-28, this nucleus eventually induces misfolding and subsequent lateral attachment of additional peptide molcules until soluble oligomers and finally insoluble fibrils are formed. We closely cooperate with the group of Gal Bitan (Department of neurology, UCLA) the tweezers’ influence on pathological protein misfolding is being investigated.
In vitro, tweezers complexation occurs selectively at lysines and arginines (NMR, MS) and prevents refolding into a β-sheet (CD). Various biophysical experiments indicate a redirection of protein aggregation from toxic Aβ oligomers and fibrils into amorphous nontoxic aggregates. Nerve cell viability is completely restored after Ab lesion (MTT, LDH) and electrophysiological studies demonstrate the same effect for miniexcitatory postsynaptic currents (mepsc) and long term potentiation (LTP). Triply transgenic mice hardly produce stainable deposites in the cortex, and initial behavioural tests were very promising (Y-Maze).
Our new molecular tweezers likewise suppress pathological aggregation of α-Synuclein (Parkinson); thus a 10 µM concentration in water almost entirely prevents deformation and premature death of transgenic zebra fish. A remarkable catalytic efficiency was found when the phosphate tweezers were added to aggregating Islet Amyloid Precursor Peptide (Diabetes mellitus II). Here, the formation of insoluble β-sheets from the extremely aggregation-prone IAPP was still effectively suppressed by a million-fold lower tweezer concentration (ThT, TEM).
Our world-wide collaboration with neurobiological groups constantly reveals new applications for molecular lysine and arginine tweezers. Here again we synthesize further developed prototypes with increased protein selectivity and affinity. With their help we investigate mechanisms of aberrant protein folding and explore new disease-modifying therapeutic strategies.
References
- Lysine-specific molecular tweezers are broad-spectrum inhibitors of assembly and toxicity of amyloid proteins. S. Sinha, D. H. J. Lopes, Z. Du, E. S. Pang, A. Shanmugam, A. Lomakin, P. Talbiersky, A. Tennstaedt, K. McDaniel, R. Bakshi, P.-Y. Kuo, M. Ehrmann, G B. Benedek, J. A. Loo, F.-G. Klärner, T. Schrader, C. Wang, G. Bitan, J. Am. Chem. Soc. 2011, 133, 16958-16969.
- A Novel “Molecular Tweezer” Inhibitor of a-Synuclein Neurotoxicity in Vitro and in Vivo. S. Prabhudesai, S. Sinha, A. Kotagiri, A. Fitzmaurice, F.-G. Klärner, T. Schrader, G. Bitan, J. M. Bronstein, Neurotherapeutics 2012, 9, 464-476.
- Protection of primary neurons and mouse brain from Alzheimer’s pathology by molecular tweezers. A. Attar, C. Ripoli, P. Maiti, S. Sinha; T. Liu, M. R. Jones; F. Yang, G. D. Gale, K. Lichti-Kaiser, C. Tseng, M. Tan, C.-W. Xie,J. L. Straudinger, F.-G. Klärner, T. Schrader, S. A. Frautschy, C. Grassi, G. Bitan, Brain2012, 135, 3735-3748.