Group 15 azides
1.2.2.2 Group 15 azides
Binary group 15 azides have been studied over a long period of time and several neutral polyazides (E(N3)3, E(N3)5; E = P, As, Sb) and ionic polyazides [E(N3)4]+ (P, As, Sb), [E(N3)4]- (As, Sb) and [E(N3)6]- (P, As, Sb) have been prepared.[1] However, structurally characterized complexes were still limited to As(N3)3, Sb(N3)3, As(N3)4-, Sb(N3)4-, Sb(N3)52-, P(N3)6-, As(N3)6-, Sb(N3)6-, and bipyE(N3)3 (E = As, Sb). The same is true for bismuth azides, for which only ([Bi(N3)4]-, [Bi(N3)5]2-, [Bi(N3)6]3-), [Bi(N3)5]2-, [(bipy)2Bi(N3)5]2-, [(bipy)2Bi(N3)3]2) have been structurally characterized. We therefore synthesized several heteroleptic organoantimony and -bismuth compounds as well as homoleptic polyazides and studied their solid state structures.
Heteroleptic Organometalazides
Amidinato-substituted antimony LSb(N3)2 (L1 = t-BuC(Ni-Pr)2; L2 = t-BuC(NDipp)2) and bismuth diazides LBi(N3)2 were synthesized by reaction of the corresponding difluorostibines LSbF2 with Me3SiN3 and by salt elimination reaction of L1BiI2 and L2BiCl2 with AgN3.[2]
The amidinate moieties in LSb(N3)2 adopt N,N'-chelating (h2) coordination modes, resulting in heavily distorted "saw horse" conformations of the central Sb atoms, in which the stereochemically active electron lone pair adopts an equatorial position.
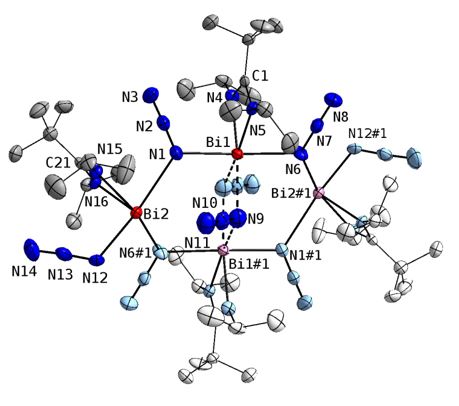
In contrast, L1Bi(N3)2 forms a centrosymmetric eight-membered Bi4N4 ring with Nα-bridging azide groups. In addition, two weak interactions between Bi(1) and Bi(1)# and the rather ionic azide moiety (N(9)-N(10)-N(11)) were observed.
Homoleptic Polyazides
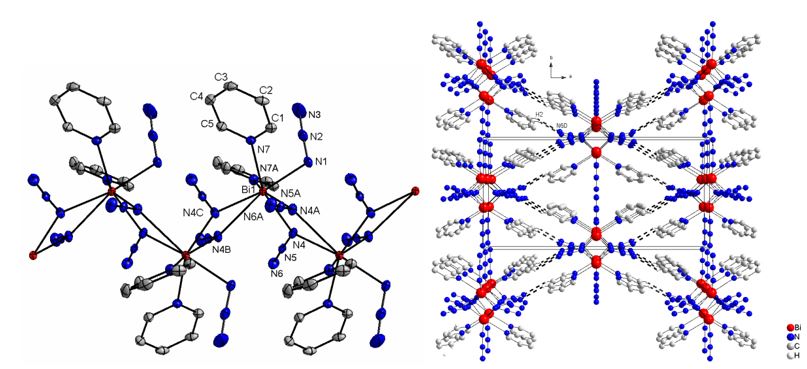
We also investigated the synthesis of homoleptic group 15 polyazides and structurally characterized group 15-triazides Sb(N3)3 as well as the base-stabilized bismuth triazide Py2-Bi(N3)3 (Py = pyridine). The Bi atom in Py2-Bi(N3)3 is coordinated by two pyridine bases and three azido ligands with that on the special position adopting a terminal binding mode. The remaining two azido groups serve as (asymetrically) bridging groups (through Na), resulting in the formation of an endless chain structure constituted by 21 symmetry. Each Bi atom adopts a sevenfold coordination geometry. The azide groups differ slightly form lineararity (N1-N2-N3 177.4(5), N4-N5-N6 176.8(2)° and the Na-Nb bonds (N1-N2 1.214(4); N4-N5 1.213(3) Å) are longer than the Nb-Ng bonds (N2-N3 1.138(5); N5-N6 1.148(3) Å). The terminal bonded azide group shows the shortest Bi-N bond length (Bi1-N1 2.277(3) Å), whereas the Bi-N bond length to the pyridine bases (Bi1-N7 2.618(2) Å) is in between those observed for the bridging azide groups (Bi1-N4 2.304(2) Bi1-N4A 2.797(2) Å). Additional intermolecular CH···N hydrogen bridges to an adjacent chain via interactions between the N6-atoms of the bridging azides and H2 atoms of the pyridine bases, resulting in the formation of a three dimensional network. C2-H2···N6D shows typical distances and angle of a non-classical hydrogen bond (H···N 2.59 Å, CH···N 128.6°).
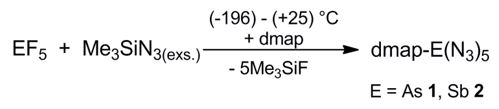
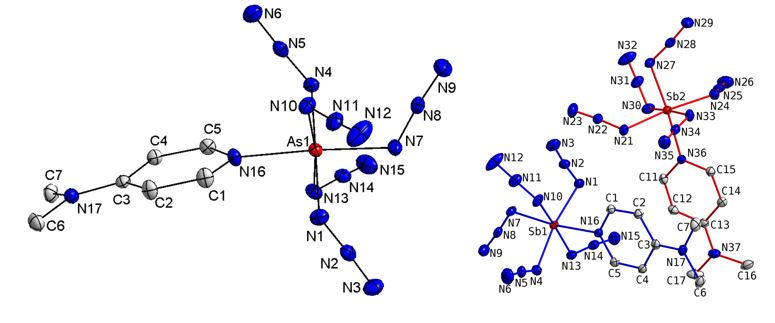
In addition, base-stabilized pentaazides dmap-E(N3)5 (E = As, Sb; dmap = 4-dimethylaminopyridine) were synthesized by reaction of equimolar amounts of freshly prepared samples of the pentaazides E(N3)5 (E = As, Sb) with dmap and structurally characterized.[3]
The N–N bond lengths within the azido ligands in both pentaazides are in the typical range observed for covalent p-block azides. The differences between the Na–Nb and Nb–Ng bond lengths, the latter are significantly shorter, clearly prove the covalent-bonded nature of the azido ligands. The azido ligands slightly deviate from linearity and show typical N–N–N bond angles of about 175°. The five E–N bond lengths toward the azido groups are almost equidistant (As: 1.9233(13) – 1.9348(13) Å; Sb: 2.0636(12) – 2.0814(12) Å).
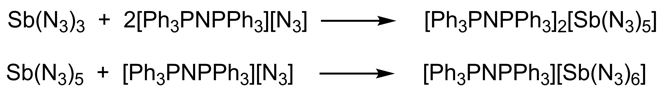
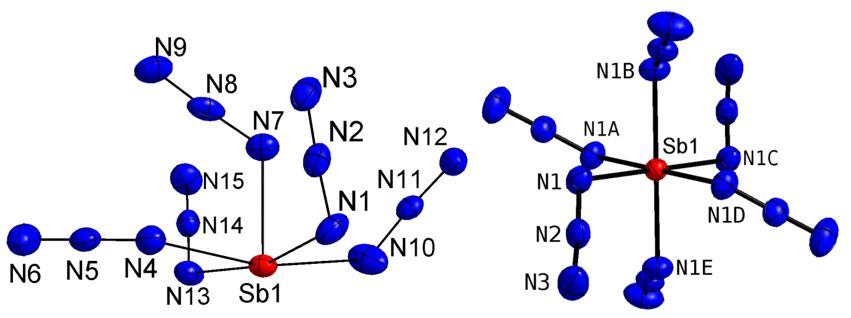
Homoleptic polyazides can also be obtained as polyanoions. We synthesized [Ph3PNPPh3]2[Sb(N3)5] containing the pentaazido dianion [Sb(N3)5]2- as well as [Ph3PNPPh3][Sb(N3)6] containing a hexaazidoantimonate anion and reported on their solid state structures.[4]
Both compounds show show strong adsorption bands due to the asymmetric ([Sb(N3)5]2-: IR: n = 2093, 2072, 2049, 2020 cm-1; Raman: n = 2097, 2076, 2053 cm-1; [Sb(N3)6]-: IR: n = 2085 cm-1; Raman: n = 2115, 2085 cm-1) and symmetric N-N-N stretching mode ([Sb(N3)5]2-: IR: n = 1297, 1160 cm-1; Raman: n = 1333, 1273 cm-1; [Sb(N3)6]-: IR: n = 1399, 1334 cm-1; Raman: n = 1336, 1262 cm-1). These experimental values also correspond well with calculated values (B3LYP and BP86 level of theory).
The [Sb(N3)5]2- dianion adopts a distorted square-pyramidal geometry as was observed for the [Te(N3)5)]- monoanion, while [Fe(N3)5)]2- containing a pentaazido dianion adopts a trigonal-bipyramidal coordination sphere. In addition, a distorted square-pyramidal coordination mode was reported for SbPh5[11] and BiPh5 [12]).[5] The equatorial Sb-N bonds (2.262(2) – 2.324(2) Å) in the [Sb(N3)5]2- dianion are significantly elongated compared to the axial Sb(1)-N(7) bond (2.099(2) Å), which is very close to the sum of the covalent radii as reported by Pyykko et al. (2.11 Å) and compared with the Sb-N bond lengths in Sb(N3)3 (2.119(4) Å; 2.119(5) – 2.151(5) Å). In contrast, the N-N bond lengths and N-N-N bond angles in the [Sb(N3)5]2- dianion are in the typical range observed for covalent azides and also comparable to the values reported for Sb(N3)3.
References
[1] For recent review articles see: a) P. Portius, M. Davis, Coord. Chem. Rev. 2013, 257, 1011; b) W. P. Fehlhammer, W. Beck, Z. Anorg. Allg. Chem. 2013, 639, 1053.
[2] a) B. Lyhs, D. Bläser, C. Wölper, S. Schulz, Chem. Eur. J. 2011, 17, 4914; b) B. Lyhs, D. Bläser, C. Wölper, R. Haack, G. Jansen, S. Schulz, Eur. J. Inorg. Chem. 2012, 27, 4350.
[3] S. Schulz, B. Lyhs, G. Jansen, D. Bläser, C. Wölper, Inorg. Chem.2012, 51, 5897.
[4] B. Lyhs, G. Jansen, D. Bläser, C. Wölper, S. Schulz, Chem. Eur. J. 2011, 17, 11394.
[5] a) P. J. Wheatley, J. Chem. Soc. 1964, 3718. b) A. L. Beauchamp, M. J. Bennett, F. A. Cotton, J. Am. Chem. Soc. 1968, 90, 6675; c) A. Schmuck, J. Buschmann, J. Fuchs, K. Seppelt, Angew. Chem. Int. Ed. 1987, 26, 1180.
For more: Group 17 azides
Go back: Group 14 azides