Bisamidinate and tetraamidinate metal complexes
Bisamidinate and tetraamidinate metal complexes
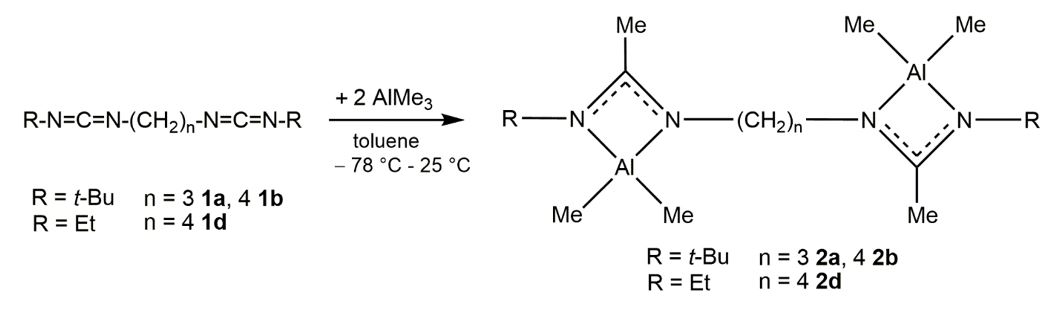
While simple amidinate complexes of transition metals and main group metals have been studied in detail, bridging biscarbodiimides and bisamidinates remained almost unknown except for a few SiMe2-bridged compounds of the type [Me2Si{NC(Ph)NDipp}2]Li2 as well as meso/rac-{CpTiMe2[N(t-Bu)C(Me)N]}2(CH2)n (n = 3, 4) and meso/rac-{CpZrMe2[N(t-Bu)C(Me)N]]}2(CH2)n (n = 4, 6, 8).[1-3] We recently reacted biscarbodiimides (R = t-Bu n = 3, n = 4; R = Et n = 4) with two equivalents AlMe3 to the corresponding bisamidinate aluminum complexes.[4]
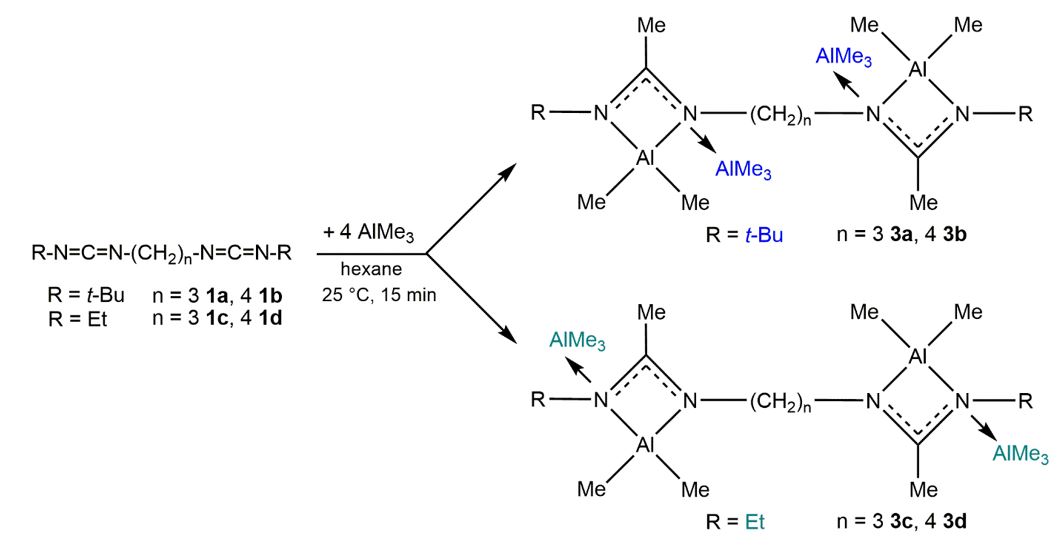
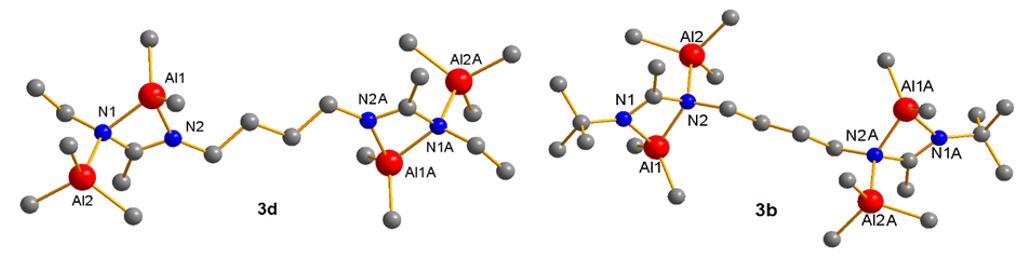
Reactions with four equivalents AlMe3 resulted in the coordination of two additional AlMe3 molecules to the "inner" imine donor while the reaction of the corresponding ethyl substituted biscarbodiimide with four equivalents of AlMe3 gave the "outer"-coordinated complex. The different coordination mode most likely results from the different steric demand of the smaller ethyl group compared to the bulkier t-butyl group.[4]
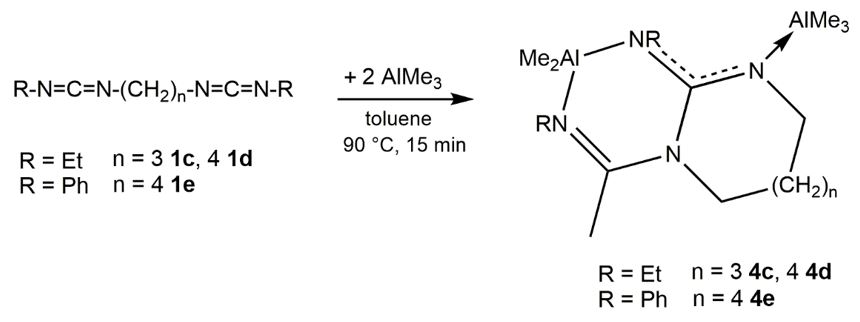
The complexes rearrange at elevated temperatures via a nucleophilic attack of the inner amidinate nitrogen atom to the electrophilic carbon atom of the second carbodiimide unit.
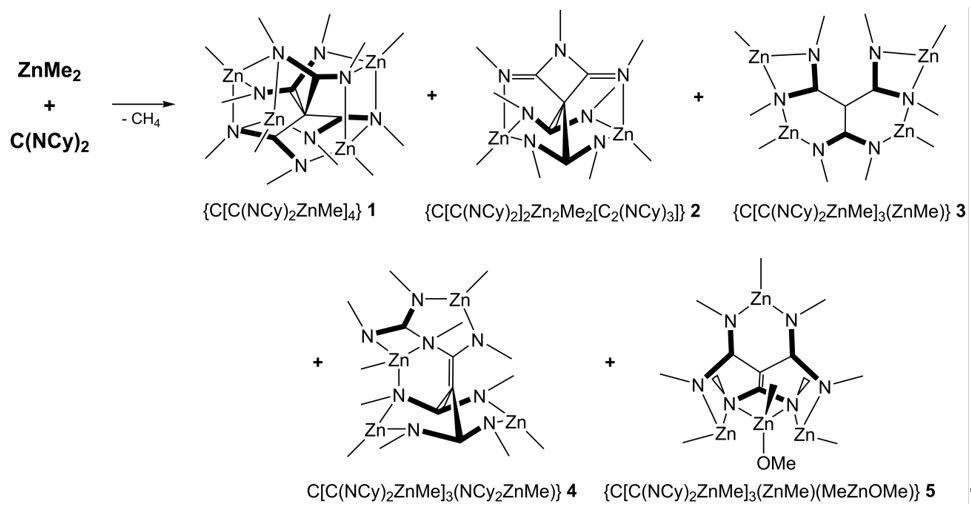
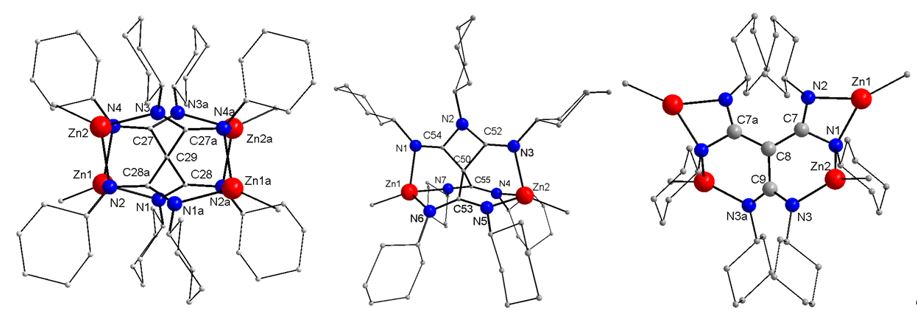
In addition, we developed a synthetic pathway to unforeseen tetraamidinate complexes by reaction of ZnMe2 with different carbodiimides (RN)2C (R = Et, i-Pr, Ph, Cy).[5]
The formation of such complexes, which are formed by C-C coupling reaction, strongly depend on the steric demand of the organic substituents of the starting carbodiimide and were only observed in reactions with ZnMe2. For instance, no reaction was observed with sterically more demanding carbodiimides (R = t-Bu, SiMe3, Dipp).
References
[1] S. D. Bai, J. P. Guo, D. S. Liu, W. Y. Wong, Eur. J. Inorg. Chem. 2006, 4903.
[2] J. R. Babcock, C. Incarvito, A. L. Rheingold, J. C. Fettinger, L. R. Sita Organometallics 1999, 18, 5729.
[3] a) W. Zhang, L. R. Sita Adv. Synth. Catal. 2008, 350, 439; b) J. Wei, W. Hwang, W. Zhang, L. R. Sita J. Am. Chem. Soc. 2013, 135, 2132.
[4] S. Schulz, M. Bayram, D. Bläser, C. Wölper Organometallics 2014,33, 2080.
[5] a) M. Münch, U. Flörke, M. Bolte, S. Schulz, D. Gudat, Angew. Chem. 2008, 120, 1535; b) S. Schmidt, S. Gondzik, S. Schulz, D. Bläser, R. Boese, Organometallics 2009, 28, 4371; c) S. Schmidt, B. Gutschank, S. Schulz, D. Bläser, R. Boese, C. Wölper, Eur. J. Inorg. Chem. 2011, 28, 4464.