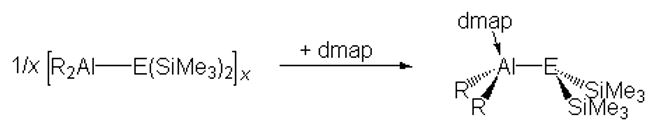Reactivity studies of group 13/15 heterocycles
Base stabilized monomeric Group 13/15 compounds
The oligomerization tendency of III/V compounds can be suppressed by using sterically demanding ligands and monomeric compounds R2MER'2 with organic substituents such as t-Bu, SiPh3 or Mes* (2,4,6-(t-Bu)3C6H2) have been synthesized. Since both central atoms M and E exhibit the coordination number 3, these compounds contain both an empty p-orbital and an electron lone pair, i.e. a Lewis acidic and Lewis basic center. This electronic structure is due to the possible formation of a double bond (dative π-bond) of great interest.[1]
Monomeric compounds can not only kinetically stabilized by using sterically-demanding ligands but also by coordination of strong Lewis bases (electronic stabilization). Thus, heterocycles of the type [R2AlE(SiMe3)2]x are cleave by the strong Lewis base 4-dimethylaminopyridine (dmap) to the corresponding base-stabilized, monomeric compounds (R = Me, Et; E = P, As, Sb, Bi).[2]
The comparison of the structural data shows a particular decrease of the sum of the bond angles at the group 15 atom (or of the average X-E-X-angle). The sum of angles steadily decrease from about 310 ° (Ø 103 °) for E = P to about 295 ° (Ø 98 °) for E = Bi.
Base-stabilized, monomeric III/V compounds are expected to be more versatile reagents than the heterocycles due to the presence of an electron lone pair of the group 15 atom, which is available for subsequent reactions such as complex formation reactions.
Reactions with transition metal complexes
Base-stabilized, monomeric group 13/15 compounds react with carbonyl complexes of various transition metals such as (Me3N)Cr(CO)5, Fe3(CO)12 and Ni(CO)4 with formation of bimetallic complexes, in which a hard main group metal M and a soft transition metal M' are bridged by the pentel atom E.[3] Such complexes were nearly unknown before.[4]
The complexes typically show relatively long bonds between the pentel and the transition metal atom. The M-E bonds of the nickel complexes are hardly influenced, whereas in the chromium and iron complexes the M-E bonds are slightly extended due to sterical interactions between the larger transition metal fragment and the relatively small phosphorus center.
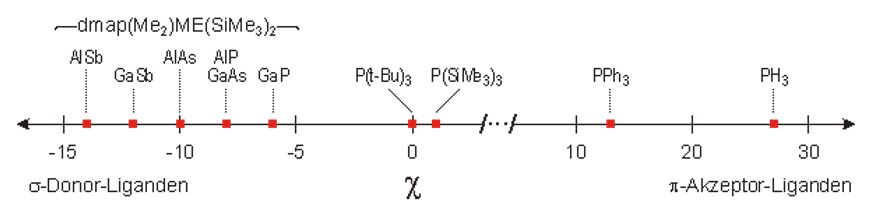
IR spectroscopic data of Ni(CO)3 complexes demonstrate in accordance with 13C-NMR measurements a clear trend in the electronic properties of the carbonyl complexes, where the Tolman parameter χ can be used to determine of the π-acceptor ability of a ligand.[7] All dmap-coordinated monomers dmap–(R2)M-ER'2 show negative χ values between -6 and -14, while for usual phosphine ligands like PPh3 or PH3 positive χ values between 10 and 30 were reported. The dmap-coordinated monomers dmap–(R2)M-ER'2 must be considered as weak π-acceptors (in the meaning of the classical model of σ-donor bonding and π-back donation). Moreover, the π-acceptor/σ-donor-relation decreases with increasing atomic number of the group 15 atom. The heavier group 15 element-transition metal bond show an increasing dative σ-bond character with almost negligible π-back-bonding content.
References
[1] P. P. Power, Chem. Rev. 1999, 99, 3463.
[2] (a) S. Schulz, M. Nieger, Organometallics 2000, 19, 2640. (b) A. Kuczkowski, F. Thomas, S. Schulz, M. Nieger, Organometallics 2000, 19, 5758. (c) F. Thomas, S. Schulz, M. Nieger, Eur. J. Inorg. Chem. 2001, 161.
[3] (a) F. Thomas, S. Schulz, M. Nieger, Organometallics 2001, 20, 2405. (b) F. Thomas, S. Schulz, M. Nieger, M. Nättinen, Chem. Eur. J. 2002, 8, 1915.
[4] (a) C. Tessier-Youngs, C. Bueno, O. T. Beachley, Jr., M. R. Churchill, Inorg. Chem. 1983, 22, 1054. (b) O. T. Beachley, Jr., M. A. Banks, J. P. Kopasz, R. D. Rogers, Organometallics 1996, 15, 5170. (c) U. Vogel, A. Timoshin, Y., M. Scheer, Angew. Chem. 2001, 113, 4541.
[5] C. A. Tolman, Chem. Rev. 1977, 77, 313.