Reactivity studies of monovalent organozinc complexes
Oxidation reactions
Since the discovery of the first Mg(I) complex by Jones et al. in 2007 numerous studys of the reductive behaviour of Mg(I) compounds were revealed.[11-13] In contrast, only two examples for the reductive behaviour of Zn(I) compounds are publicated.
The first oxidation reactions of a Zn(I) compound were performed by Yang et al..[14] The Zn(I) compound reacts with phenyl acetylene in different ratios to the zinc acetylene dimer, respectively to the zinc diacetylene (Scheme 7).
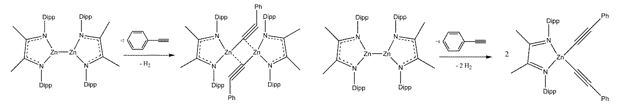
Scheme 7: Reactions of a Zn(I) compound with phenyl acetylene.
Furthermore Zn(I) compounds react with alkyl R-X (R= Me, Et; X= Br, I) to zinc alkyl complexes and the corresponding zinc halogenid dimers (Scheme 8).[15]
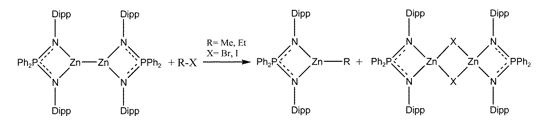
Scheme 8: Reaction of a Zn(I) compound with alkyl halides.
Reactions with azides
Our general interest in the redoxactivity of low-valent organozinc complexes prompted us to study reactions of Mesnacnac2Zn2 with group 14 azides RN3 (R = Ph, Dipp) and Me3MN3 (M = Si, Sn). The reaction of 2 eq of Me3MN3 (M= Si, Sn) with Mesnacnac2Zn2 yielded hexamethyldisilane and -distannane Me6M2 as well as the previously reported zinc azido complex [MesnacnacZnN3]2 (Scheme 9).
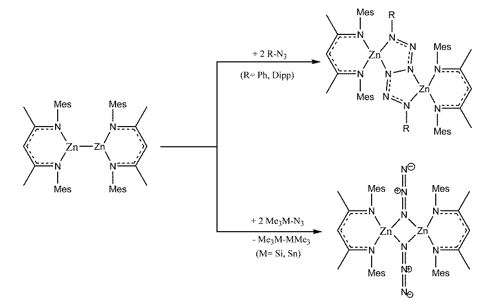
Scheme 9: Reactions of Mesnacnac2Zn2 with organic and inorganic azides.
However, Mesnacnac2Zn2 reacts with 2 eq Ph-N3 and Dipp-N3 under reductive N-N coupling to the first zinc hexazene complex (MesnacnacZn)2(μ-η2:η2-N6Ph2) with a central Ph2N6 unit, which adopts a bridging position and is connected to two four-coordinate Zn atoms (Scheme 9).[16] Analogue reactions of Dippnacnac2Zn2 with 2 eq PhN3 lead to the hexazene complex (DippnacnacZn)2(μ-η2:η2-N6Ph2). The crystal structures are shown in Figure 5.
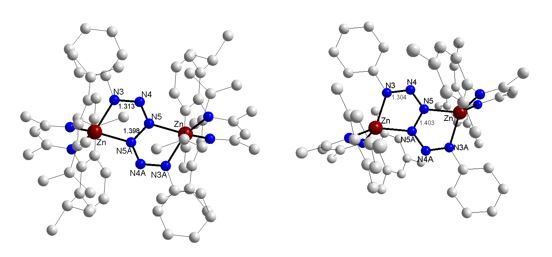
Figure 5: Molecular structures of (DippnacnacZn)2(μ-η2:η2-N6Ph2) (left) and (MesnacnacZn)2(μ-η2:η2-N6Ph2) (right).
The different N–N bond lengths within the hexazene unit give insight into the electron localization within the complex. The central N5–N5A bond (1.403 Å) is significantly elongated compared to the almost identical N3–N4 (1.3041 Å) and N4–N5 (1.3012 Å) bonds, hence the hexazene unit is best described as a dianionic ligand with a central N5–N5A single bond and two allyl-like, monoanionic N3 moieties containing delocalized p-electrons between N3, N4 and N5. The hexazene unit serves as a dianionic ligand, which agrees with the description of the Zn atoms in the formal oxidation state +II. Four resonance structures of the dianionic hexazene unit can be drawn (Figure 6).
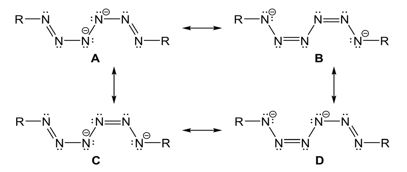
Figure 6: Resonance structures of the hexazene ligand.
The resonance structures C and D are identical. A is less reasonable due to the neighbouring negatively charged N-atoms. The resonance structures with a central N–N single bond and delocalized p-electrons over the terminal N3 units perfectly agrees with the experimentally observed N–N bond distances
Hexazene complexes are still rare and only three magnesium[17] und four iron hexazene[18-20] complexes have been structurally characterized, to date.
